Next: Immunity Up: VECTRI Description Previous: Indoor Temperatures
One of the new aspects of the VECTRI model is that is explicitly
allows the interaction between vector and host population on a
district and regional scale. The VECTRI model specifies the
population density ![]() using the Africa-only AFRIPOP [#!linard:12a!#]
or global GRUMP [#!balk:06!#] datasets which have a nominal 1 km and 4.5 km spatial
resolution, respectively. Thus at each location (model spatial grid
cell), the ratio of biting vectors to hosts is known and is given by
(
using the Africa-only AFRIPOP [#!linard:12a!#]
or global GRUMP [#!balk:06!#] datasets which have a nominal 1 km and 4.5 km spatial
resolution, respectively. Thus at each location (model spatial grid
cell), the ratio of biting vectors to hosts is known and is given by
(
![]() ). This is important to represent
the vector-to-host transmission rate. The number of bites
). This is important to represent
the vector-to-host transmission rate. The number of bites ![]() that
any particular individual receives in a given time the human biting
rate (
that
any particular individual receives in a given time the human biting
rate (![]() ) is considered to be a random process, and thus
distributed following a Poisson process with a mean biting rate of
) is considered to be a random process, and thus
distributed following a Poisson process with a mean biting rate of
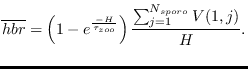 |
(12) |
The daily number of infectious bites by infectious vectors, ![]() ,
is the product of
,
is the product of ![]() and the CircumSporozoite Protein Rate,
and the CircumSporozoite Protein Rate, ![]() .
Specifically in the VECTRI notation, this is
.
Specifically in the VECTRI notation, this is
![]() , with
the
, with
the ![]() indicating that the calculation is restricted to the vectors
that are biting within the present timestep of the model. This implicitly assumes
that there is no change in the intensity of biting or the gonotrophic
cycle length between uninfected and infectious vectors; a
simplification according to [#!koella:98!#]. If the transmission
probability from vector to host for a single bite of an infective
vector,
indicating that the calculation is restricted to the vectors
that are biting within the present timestep of the model. This implicitly assumes
that there is no change in the intensity of biting or the gonotrophic
cycle length between uninfected and infectious vectors; a
simplification according to [#!koella:98!#]. If the transmission
probability from vector to host for a single bite of an infective
vector, ![]() , is assumed a constant (VECTRI adopts a value of 0.3
[#!ermert:11a!#]) then the transmission probability for an individual
receiving
, is assumed a constant (VECTRI adopts a value of 0.3
[#!ermert:11a!#]) then the transmission probability for an individual
receiving ![]() infectious bites will be
infectious bites will be
![]() . The impact
on transmission due to blocking immunity is neglected. Thus the
overall transmission probability per person per day in the model can
be obtained by integration the over the bite distribution:
. The impact
on transmission due to blocking immunity is neglected. Thus the
overall transmission probability per person per day in the model can
be obtained by integration the over the bite distribution:
![[*]](crossref.png) could
be modified to incorporate this, increasing the mean bite rate for a
subset of the unprotected population. This involves a number of
complications however, since accurate data would be required
concerning bednet distribution and use, how this usage correlates to
host infective state, and which proportion of bite are taken during
the hours of sleeping.
could
be modified to incorporate this, increasing the mean bite rate for a
subset of the unprotected population. This involves a number of
complications however, since accurate data would be required
concerning bednet distribution and use, how this usage correlates to
host infective state, and which proportion of bite are taken during
the hours of sleeping.
There is a differential mean bite rate for hosts in the exposed, infected and recovered (EIR) individuals relative to the susceptible category (S), to produce over dispersive biting rates and reflect the fact that some individuals are more attractive to vectors [#!lindsay:93!#,#!knols:95!#,#!mukabana:02!#], are more vulnerable due to clothing and housing standards[#!lwetoijera:13!#], access to nets, location of housing with respect to water bodies [#!carter:00!#,#!bousema:12!#,#!kienberger:14!#], and that parasite infection also appears to increase attractiveness of individuals to vectors [#!lacroix:05!#], although the latter effect is offset by increased net use in the case of clinical symptoms. anthrophic vectors
The impact of using eqn. ![[*]](crossref.png) is to
reduce the mean transmission rate, particularly when the mean bite
rate is small resulting in a strong positive skewness of the Poisson
distribution (Fig. 3). While this is an
improvement on the simple assumption that all hosts receive equal
numbers of bites, the Poisson distribution is likely
under-dispersive compared to reality, since a number of factors such
as unequal host attractiveness to vectors and nocturnal behaviour
affecting exposure will likely lead to a uneven distribution of bites
rates [#!dye:86!#,#!knols:95!#,#!mukabana:02!#]. Fig. 3 also emphasizes that the model is
relatively insensitive to the choice of
is to
reduce the mean transmission rate, particularly when the mean bite
rate is small resulting in a strong positive skewness of the Poisson
distribution (Fig. 3). While this is an
improvement on the simple assumption that all hosts receive equal
numbers of bites, the Poisson distribution is likely
under-dispersive compared to reality, since a number of factors such
as unequal host attractiveness to vectors and nocturnal behaviour
affecting exposure will likely lead to a uneven distribution of bites
rates [#!dye:86!#,#!knols:95!#,#!mukabana:02!#]. Fig. 3 also emphasizes that the model is
relatively insensitive to the choice of ![]() for values exceeding
around 0.2.
for values exceeding
around 0.2.
The host population is represented by the vector
![]() , and
each VECTRI timestep a proportion
, and
each VECTRI timestep a proportion
![]() of hosts
become infected and progress through the array until 20 days later
they assume an infective status, an average value for immune and
non-immune subjects
[#!shute:51!#,#!miller:58!#,#!hawking:71!#,#!day:98!#].
Non-immune hosts clear infections at an e-folding rate of
of hosts
become infected and progress through the array until 20 days later
they assume an infective status, an average value for immune and
non-immune subjects
[#!shute:51!#,#!miller:58!#,#!hawking:71!#,#!day:98!#].
Non-immune hosts clear infections at an e-folding rate of
![]() days. Even after a century of study of the disease, the paradigm of
naturally acquired immunity (NAI) is still hotly debated [#!doolan:09!#].
Therefore the present version of the model neglects host immunity, and
the impact of the various representations of immunity in VECTRI will
be the subject of a companion article.
days. Even after a century of study of the disease, the paradigm of
naturally acquired immunity (NAI) is still hotly debated [#!doolan:09!#].
Therefore the present version of the model neglects host immunity, and
the impact of the various representations of immunity in VECTRI will
be the subject of a companion article.
PARAMETER TABLE